Studies For You
Researcher FAQs
The Basics
Studies For You is a recruitment tool to inform the public about research opportunities at the University of Utah. If you complete the information as part of your ERICA application, your study will be displayed on the University’s research website, your physician profile page, and other linked University of Utah webpages.
Yes.
The Studies For You registration form is part of the University of Utah Institutional Review Board (IRB) application. The posting goes live once the study is approved in the ERICA system.
- As part of a New Study Application. When answering the IRB application question about recruitment as part of a new study application, select the option to have the study posted to the University of Utah under "Studies For You". Then complete the University of Utah "Studies For You" page that follows in the IRB application. The IRB reviews this recruitment method as part of its normal review. The posting goes live once the study is approved in the ERICA system.
- Add "Studies For You" during a Continuing Review (CR). This is done by checking the box on Page 5 of the continuing review, question 1. 'Are there any changes to be made now?' Check 'Yes', then on the description of the amendment, add "Studies For You" as a recruitment tool.
- Add Studies For You as part of an amendment.
By selecting a Specialty and/or Sub-specialty in ERICA, the posting will not only be linked to the "Studies for You" website but also to the specialty webpages across the University of Utah Health websites and the researchers' profile. When the posting is deactivated on the "Studies For You" website, it will be deactivated across these specialty and researcher webpages as well.
For questions about the specialty list and the University of Utah Health webpages, contact hscwebmaster@hsc.utah.edu.
Making Changes to Live Postings
- Submit an amendment to the IRB and de-select the University of Utah "Studies For You" Posting as a recruitment option. Once the amendment is approved, the posting is deactivated automatically.
- Note that when a study submits a continuing review indicating enrollment is no longer open, the posting is also automatically removed.
Submit an amendment to the IRB and edit the information on the University of Utah "Studies For You" page. Once the amendment is approved, the posting is updated automatically.
When a study submits a continuing review indicating enrollment is no longer open, the posting is automatically deactivated and removed from the "Studies For You" website.
- When a study’s IRB approval expires or is suspended, the posting is automatically deactivated and removed from the "Studies For You" website. When IRB approval is reinstated, the posting is automatically activated again.
- When a study is closed, the posting is automatically deactivated and removed from the "Studies For You" website.
Yes, you can!
There is no additional IRB approval required to share the "Studies For You" website with patients.
Yes.
You can share the link with your patients and other providers. If you intend to formally notify your patients or providers of this posting in writing with a letter or email, this must be described in your IRB application as part of your recruitment strategy.
The automatic creation of Amendments (AM) to attach completed Studies For You translations makes the process more efficient. Approved study summary translations qualify for automatic submission by the ERICA system.
To utilize this service:
- Navigate to your study workspace and select the ‘Link Studies For You Translations’ activity.
- This activity will automatically attach your Language Translation and submit the Amendment to the IRB on your behalf.
- If you would like to include additional changes on an amendment while linking your Studies For You translation, please follow the instructions below:
The How and Why of Adding Translated Summaries
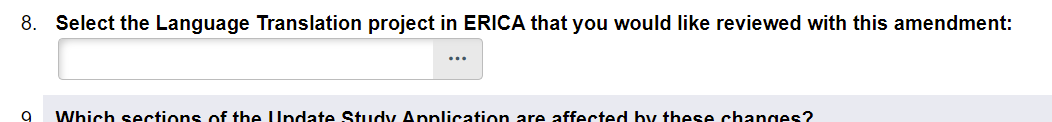
- On Page 5 of the Continuing Review, Question 1: ‘Are there any changes to be made now?’ should be set to ‘Yes’.
- On the ‘Description of Amendment’ page, under amendment type, select the ‘Language Translation via the ERICA system’ option.
- On the same page, a new question will appear to select the Completed translation. Use the ellipsis to pull up a list of translations that can be linked to this study. Please select the most recent translation.
- Finish completing the CR, including any additional changes and then submit.
- This will then be processed through to the IRB.
- The updated translated language should appear on the website after the IRB has completed their review process.
- Create an Amendment from the Parent IRB workspace.
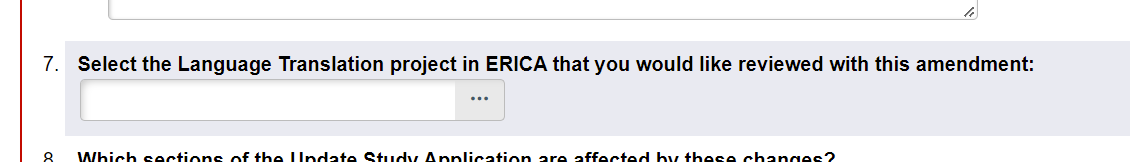
- On Page 1 of the AM, under Amendment Type, select the ‘Language Translation via the ERICA system’ option.
- Click Continue.
- On Page 2, a new question will appear to select the Completed translation. Use the ellipsis to pull up a list of translations that can be linked to this study. Please select the most recent translation.
- Finish completing the AM, including any additional changes and then submit.
- This will then be processed through to the IRB.
- The updated translated language should appear on the website after the IRB has completed their review process.
